On March 20, 2025, the U.S. Food and Drug Administration (FDA) announced its intention to extend the compliance date for the Food Traceability Rule, established under Section 204(d) of the Food Safety Modernization Act (FSMA), by 30 months. This shifts the deadline from January 20, 2026, to July 20, 2028. While this extension provides additional time for food businesses to align their operations with the enhanced traceability requirements, it does not alter the rule’s core mandates. The FDA emphasizes that the extension aims to ensure complete coordination across the supply chain, allowing all covered entities to fully implement the final rule’s requirements.
Understanding FSMA 204: Core Requirements
FSMA 204 mandates that entities involved in manufacturing, processing, packing, or holding foods listed on the Food Traceability List (FTL) maintain detailed records of Key Data Elements (KDEs) associated with specific Critical Tracking Events (CTEs).
Critical Tracking Events (CTEs):
- Harvesting
- Cooling (before initial packing)
- Initial packing of raw agricultural commodities
- First land-based receiving of food obtained from a fishing vessel
- Shipping
- Receiving
- Transformation of the food
Key Data Elements (KDEs):
- Product description
- Quantity and unit of measure
- Location descriptions for immediate previous sources and subsequent recipients
- Dates of receipt and shipping
- Traceability lot codes
These requirements aim to enhance the ability to trace foods through the supply chain rapidly, thereby improving the speed and accuracy of response during foodborne illness outbreaks.
Implications of the Extended Timeline
The extension to July 20, 2028, offers food businesses additional time to:
- Assess Current Systems: Evaluate existing traceability systems and identify gaps in capturing required KDEs.
- Engage Supply Chain Partners: Collaborate with suppliers and distributors to ensure seamless data sharing and compliance across the supply chain.
- Invest in Technology: Implement or upgrade digital systems capable of real-time data capture and reporting to meet the 24-hour record retrieval requirement.
- Develop Comprehensive Traceability Plans: Document processes for maintaining records, assigning traceability lot codes, and identifying foods on the FTL.
However, it’s crucial to recognize that the delay does not eliminate the risks associated with food safety. The issues that prompted FSMA 204, rising foodborne illness, recalls, and supply chain disruptions, remain pressing concerns. Proactive engagement with supply chain partners, investment in appropriate technologies, and the development of comprehensive traceability plans are essential steps toward compliance.
Leveraging AuditComply for FSMA 204 Compliance
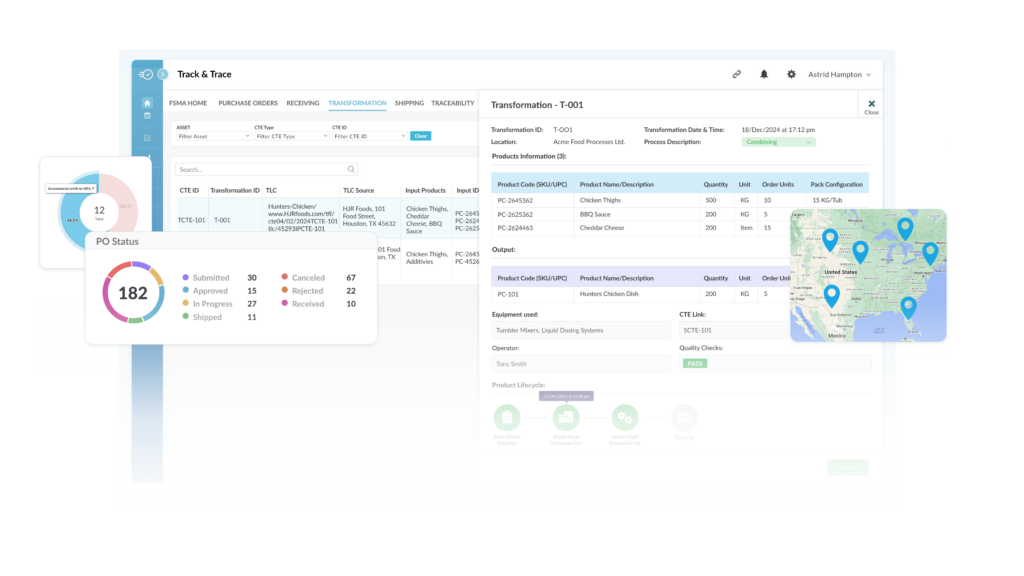
AuditComply offers a robust traceability platform designed to assist food businesses in meeting FSMA 204 requirements efficiently. Key features include:
- CTE Automation: Streamlines documentation processes by automating the capture of KDEs at each critical tracking event.
- Real-time Reporting: Enables instant retrieval and sharing of traceability records, ensuring readiness for FDA audits and rapid response during recalls.
- Supplier Compliance Management: Facilitates monitoring and verification of supplier adherence to traceability standards, promoting a cohesive compliance strategy across the supply chain.
- Customizable Traceability Plans: Assists in developing and maintaining detailed traceability plans tailored to specific operational needs and regulatory requirements.
By integrating AuditComply’s traceability solutions, food businesses can enhance transparency, ensure regulatory compliance, and strengthen their overall food safety management systems.
The extension of the FSMA 204 compliance deadline to July 20, 2028, provides a critical window for food businesses to bolster their traceability systems. Proactive engagement with supply chain partners, investment in appropriate technologies, and the development of comprehensive traceability plans are essential steps toward compliance. Leveraging platforms like AuditComply can streamline this process, ensuring that businesses not only meet regulatory requirements but also enhance their capacity to protect public health through improved food safety practices.
About AuditComply
AuditComply is a leading enterprise technology platform designed to mitigate risk, drive compliance, and enhance quality assurance and supplier management for highly regulated industries—all within a single, connected solution. By streamlining operations, delivering deep insights, and accelerating digital transformation, AuditComply empowers organizations to improve efficiency, strengthen resilience, and ensure compliance with confidence.
If you’re facing similar challenges and want to learn more about how AuditComply can transform your Risk, Quality, Compliance, and Supplier operations, reach out to us at: info@auditcomply.com, https://www.auditcomply.com/.